Introduction
Thermodynamics, in a literal
sense, is a branch of physics which deals with the relation between heat and
mechanical energy. Its foundations were, in fact, laid by experiments in which
mechanical energy was converted into heat, as in the researches. Rumford and
Joule and conversely heat converted into work as in the steam engines
introduced between the times of Rumford and Joule. Broadly speaking,
thermodynamics deals with the transformation of heat energy to other forms of
energy, such as electrical, magnetic and radiant etc.
Scope of thermodynamics
The object of the science of
thermodynamics is the study of all naturally falls under two headings, the
study of energy relations and the study of the manner in which energy changes take
place. The guiding principles for the two cases are the first and second law of
thermodynamics.
 |
| Carnot |
 |
| William Thomson |
The first law of
thermodynamics (Joule, 1843) states the connection between heat and mechanical
work and leads up to the principle of conservation of energy. The second law of
thermodynamics (Carnot, 1824, Clausius, 1850 and W.Thomson, 1851) is concerned
with the convertibility of energy and gives us a method for determining how the
amount of work obtainable from a certain quantity of heat depends on the temperatures
between which the operation takes place. It provides a mode of defining the
absolute or thermodynamic scale of temperature. These two laws from the basis
of the classical thermodynamics and from these two laws a large number of
problems on heat may be solved. In 1906, Nernst published a new theory which
states that the absolute zero of temperature is unattainable. This is now known
as the third law of thermodynamics.
Thermodynamics equilibrium
A body or system is said to
be in thermodynamic equilibrium when it is in equilibrium from the mechanical,
thermal and chemical points of view. Mechanical equilibrium means that there is
no unbalanced force either in the interior of the system or between the system
and its surroundings. Thermal equilibrium means that all the parts of the body
or system are at the same temperature as that of the surroundings. When a body
or system does not undergo any changes or chemical composition it is said to be
in chemical equilibrium.
Indicator diagram
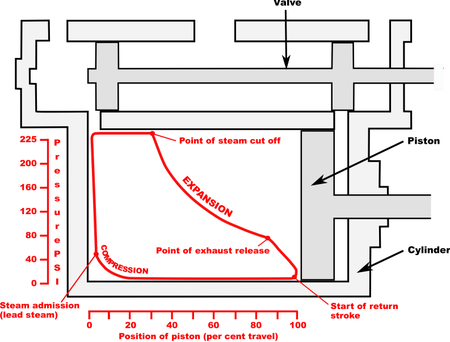 |
| Indicator diagram |
 |
| James Watt |
The method of representing
the work done during a cycle is due to James
Watt, who invented an instrument called the indicator, by means of which an engine can automatically draw a
diagram representing pressure changes in relation to volume changes. Such a
diagram, for an actual machine is accordingly called an indicator diagram, but should be distinguished from the
pressure-volume diagram which unlike an indicator diagram, relates to a
constant mass of a substance.
Internal energy or intrinsic energy
If a substance absorbs a
certain quantity of heat without any performance of external work, all the heat
goes to increase the store of energy within the substance. This store of energy
in the internal energy of the substance. In practice, it is not possible to
measure the internal energy but changes in internal energy can be readily
measured. An increase in the internal energy can be brought about by the
communication of energy to the body either as heat or by the performance of
work on the body. We use the symbol u
to denote internal energy.
The internal energy of a
body in a given state depends upon the configuration and motion of the
molecules. Thus internal energy may be regarded partly as potential energy and
partly as kinetic energy arising out of the fact that the ultimate particles of
the body are in a state of invisible chaotic motion. As no mutual force of
attraction or repulsion is exerted by the ultimate particles of a perfect gas,
all the energy is kinetic. Experimentally, Joule also proved that for a perfect
gas the internal energy is solely kinetic and is a function of temperature only
(Mayer’s hypothesis).
The change in internal energy of a body between two
different states depends only upon the initial and final states and not upon
the process by which the change is effected.
Energy equivalent of the first law of thermodynamics
The heat supplied to a
system will be spent in two ways:
(1) in doing external work and
(2) in changing the internal energy of the system. That
is,
dQ=dW+du, expressing all the quantities in work’s unit, where
dQ is the quantity of heat given to a
system, du is the change in the
internal energy and dW is the
external work done.
It may be pointed out that the expression of dQ in work’s unit can be found by
multiplying the heat supplied in calories by J, the mechanical equivalent of heat. Accordingly, the first law of
thermodynamics may be stated as follows: “The change in the internal energy of
any system during a physical process is equal to the algebraic sum of the heat
communicated to the system and the work done by external agencies on the
system, both being expressed in the same unit” (Clausius).
The quantity du
is defined with the help of principle of conservation of energy by considering
a thermally insulated system for which du=-dW,
since dQ=0. Changes in the internal
energy can thus be measured in terms of the external work done on the system.
In the case of a simple gas expanding against a uniform external pressure P, dW=PdV,
where dV is the change in volume.



No comments:
Post a Comment