Fusion and
solidification
The physical state, in which a substance exists at ordinary
temperatures, can be changed by heating or cooling. When a solid is heated it
melts into liquid and on being further heated the liquid passes into vapor.
Conversely, when a vapor is cooled it condenses to form a liquid and a liquid
on cooling solidifies. When a substance changes from the solid to the liquid
state, the process is known as fusion or
melting, and when it changes from the liquid to the solid state the process
is called solidification or freezing.
 Melting
point: When a solid is heated its temperature
gradually rises and at a certain temperature it begins to melt or turn into
liquid. This temperature remains constant if the on it remains constant
throughout the process of melting and is called the melting point and the heat absorbed during fusion is utilized for
the change of state.
Melting
point: When a solid is heated its temperature
gradually rises and at a certain temperature it begins to melt or turn into
liquid. This temperature remains constant if the on it remains constant
throughout the process of melting and is called the melting point and the heat absorbed during fusion is utilized for
the change of state. Freezing
point: A liquid when gradually
cooled begins to freeze or solidify at a particular temperature which is
different for different substances. During freezing the though cooled will not
fall in temperature. The temperature that remains constant throughout the
process of solidification is called the freezing
point and for each substance it slightly varies when the super incumbent
pressure varies. In fact, the melting point of a solid is the same as the
freezing point of the corresponding liquid.
Freezing
point: A liquid when gradually
cooled begins to freeze or solidify at a particular temperature which is
different for different substances. During freezing the though cooled will not
fall in temperature. The temperature that remains constant throughout the
process of solidification is called the freezing
point and for each substance it slightly varies when the super incumbent
pressure varies. In fact, the melting point of a solid is the same as the
freezing point of the corresponding liquid.
Latent heat
Some heat is absorbed or liberated when a substance
changes from one state to another. It may be observed that when heat is
continuously given to a solid its temperature rises till at a certain
temperature it begins to melt. If further heat is supplied, melting continues,
the temperature remaining constant. The heat supplied at this stage does not produce
a rise in temperature and the heat absorbed by the solid at the melting point
is utilized break the potential energy of molecular binding in solids. Heat
thus utilized brings about a change of state at constant temperature and Black
called this as latent heat. When a
liquid solidifies this heat is given out. The word latent means hidden; so
latent heat means that heat for which there is no external manifestation, such
as rise or fall of temperature.
Liquids of evaporation absorb similar heat at the
boiling point and pass into vapor without change of temperature; latent heat is
absorbed by the liquid to fight against the molecular binding forces in liquid
which is almost absent in vapor molecules.
The latent heat of fusion of a solid may be defined as
the quantity of heat required to change unit mass of the substance at its
melting point from the solid to the liquid state without any change of
temperature. The same quantity of heat is also given out by unit mass of
substance at the same temperature in changing from the liquid state to the
solid state without any change of temperature. The statement that latent heat
of fusion of ice is 80 calories indicates that 1 gm of ice at 0°C requires 80
calories of heat to be converted to 1 gm of water at 0°C. Conversely, 80
calories of heat are given out when 1 gm of water at 0°C changes to 1 gm of ice
at 0°C.
Sensible
heat
Sensible is that which brings about a change of
temperature when given to a substance and is, therefore, accountable. But
latent heat is only absorbed or liberated at the time of change of state
without change of temperature.
According to the kinetic theory, temperature of a
substance is measured by the kinetic energy of its molecules. Sensible heat
increases the kinetic energy of its molecules. Sensible heat increases the
kinetic energy of the molecules, while latent heat increases the potential
energy of the molecules.
Laws of
fusion
(1)
Every
(crystalline) solid melts at a definite temperature. This temperature is called
the melting point or fusion point of the solid. It is also
the freezing point or solidification point of the corresponding liquid. When
the pressure is one atmosphere, the melting point is called its normal melting
point.
(2)
The rate at
which fusion takes place is proportional to the rate of supply of heat, but
temperature remains constant until the whole of the solid melts.
(3)
In order to melt
unit mass of a solid into its liquid at the same temperature, a definite
quantity of heat, known as the latent
heat of fusion of the solid, must be given to it. This latent heat of solid
varies very little with the pressure on its surface.
(4)
During fusion
there is always a change in volume. For solids like paraffin wax, ghee, copper,
etc. which expand on melting, an increase of pressure raises the melting point,
while for solids like ice, iron, antimony, bismuth, which contract on melting,
an increase of pressure lowers the melting point.
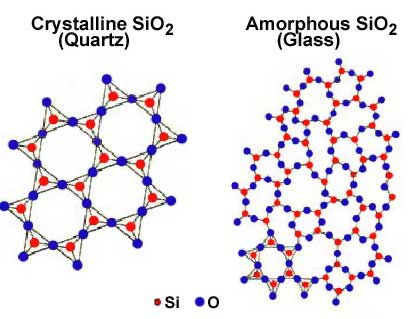
Crystalline
solids and amorphous solids
Crystalline solids have regular geometrical shape.
Most solids belong this class. Ice, sugar, copper sulphate, common salt,
metals, etc. are crystalline in structure. Only crystalline substances have a
definite melting point.
Amorphous solids have no regularity of shape- they
are structureless solids. Glass, pitch, etc. belong to this class. Amorphous
substances change first from the solid to the plastic state, and then to the
liquid state. The plastic state exists over a long range of temperature.



No comments:
Post a Comment